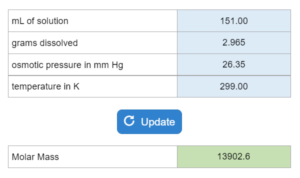
Tutor – Using Osmotic Pressure to Determine Molar Mass
In the lab a student finds a solution with x mL of water contains x grams of a compound. Osmotic pressure (mm Hg) and temperature (K) are also given. Determine the molar mass.
Experts Have Solved This Problem
Please login or register to access this content.