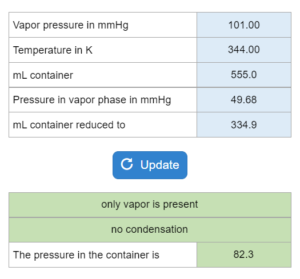
Mastery – Vapor Pressure: Evaporation or Condensation – Choose all that apply
Choose all that apply. A liquid's vapor pressure (in mmHg)at a temperature (K) is given. The compound is placed in a container (mL) and put at a certain pressure (mmHg). If the container is reduced to a new mL, what happens?
Experts Have Solved This Problem
Please login or register to access this content.