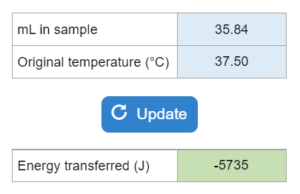
The volume (mL) and temperature (°C) for a sample of benzene are given along with its melting point, density, specific heat and fusion enthalpy. How much energy is transferred in J?
Experts Have Solved This Problem
Please login or register to access this content.