Tutor – Effect of Temperature Change on Solubility
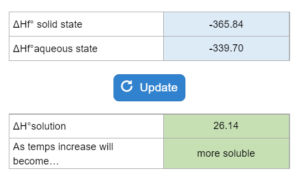
The ΔHf° for an aqueous and solid-state of a salt are given. What is the ΔH° solution and will the salt be less or more soluble with increasing temperatures.
Experts Have Solved This Problem
Please login or register to access this content.