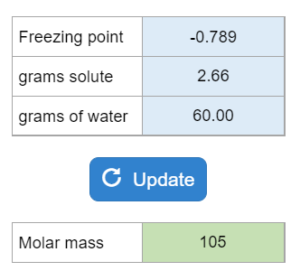
Tutor – Determining Molar Mass from Freezing Point Depression (Positive Kf)
A solution made with a nonelectrolyte has a given freezing point. Grams of solute and water are given. What is the molar mass of the solute?
Experts Have Solved This Problem
Please login or register to access this content.