Tutor – Determining Enthalpy of Vaporization from Temperature and Pressure
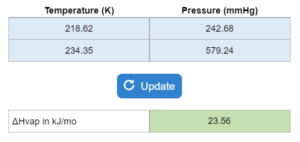
Two temperatures (in K) and two pressures are given. Determine the enthalpy of vaporization (ΔHvap).
Experts Have Solved This Problem
Please login or register to access this content.