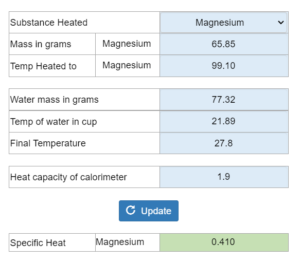
Tutor – Coffee Cup Calorimetry: Specific Heat I
Coffee Cup Calorimeter. X grams of a substance is heated and put into a coffee cup with water. Final temperature and heat capacity of the calorimeter are given. What is the specific heat of the substance?
Experts Have Solved This Problem
Please login or register to access this content.