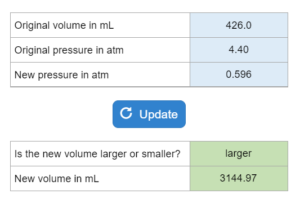
A gas sample is given with an original volume (in mL) and pressure (in atm). The new pressure is also given, what is the new volume in mL? Is the new volume larger or smaller than the original?
Experts Have Solved This Problem
Please login or register to access this content.