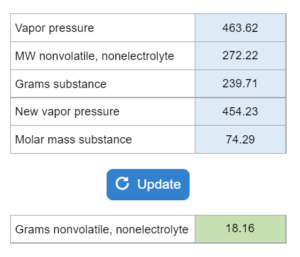
Mastery – Raoult’s Law Calculations: The Vapor Pressure…
The vapor pressure of a substance is given as well as the g and MW of a nonvolatile, nonelectrolyte. How many grams of the non-electrolyte need to be added to the substance to reduce the vapor pressure to x. Molar mass of the substance is also given.
Experts Have Solved This Problem
Please login or register to access this content.