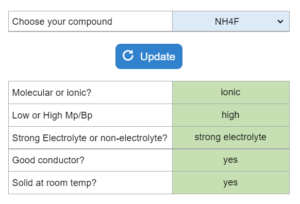
A compound is given. Determine which answers are true. Low or high melting point? Strong electrolyte or non-electrolyte? Ionic or molecular? Good conductor? Solid at room temperature?
Experts Have Solved This Problem
Please login or register to access this content.