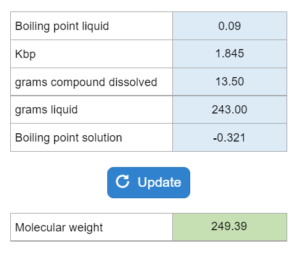
Mastery – Molar Mass from Boiling Point or Freezing Point
The boiling point or freezing point of a liquid is given as well as the Kb. X grams of an unknown compound are dissolved in x grams of liquid creating a new boiling or freezing point. Calculate molecular weight for the unknown compound. NOTE: this solver works whether you are given boiling points OR freezing points.
Experts Have Solved This Problem
Please login or register to access this content.