Mastery – Intermolecular Forces and Vapor Pressure
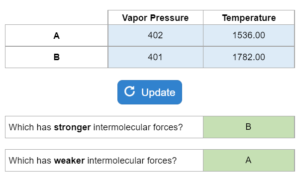
A chart is given with compounds A & B, their vapor pressures and temperatures. This solver determines the stronger and/or weaker intermolecular forces, if the vapor pressure would be higher or lower at a new temperature and which has the highest vapor pressure at a certain temperature.
Experts Have Solved This Problem
Please login or register to access this content.