Mastery – Heat Transfer: Phase and Temperature Changes (How many kJ of energy?)
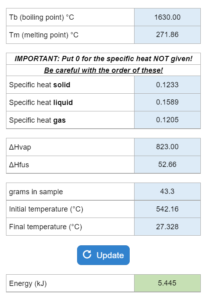
The Tb, Tm, specific heats (gas, liquid, and/or solid) ΔHvap and ΔHfus are given. A sample is given with a mass in grams and initial temperature and final temperature. How much energy in Joules are needed? This solver works for rising or decreasing temperatures.
Experts Have Solved This Problem
Please login or register to access this content.