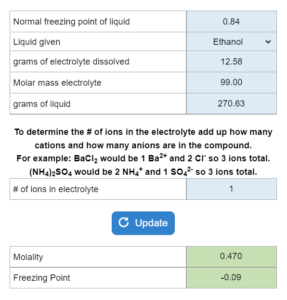
Mastery – Electrolyte Solutions: Boiling/Freezing Point Calculations, Molality and Freezing Point
The freezing point of a liquid is given. An electrolyte is dissolved (grams and molar mass given) in x grams of the liquid. There is also a table of Kb and Kf values given. What is the molality and what is the freezing point?
Experts Have Solved This Problem
Please login or register to access this content.