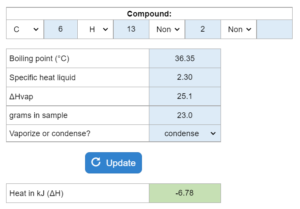
The boiling point, specific heat liquid and ΔHvap are given. Determine how many kJ of heat (ΔH) are needed to vaporize or condense x grams at 1 atm of pressure. This solver works for both questions asking for vaporizing the sample and questions asking to condense the sample.
Experts Have Solved This Problem
Please login or register to access this content.