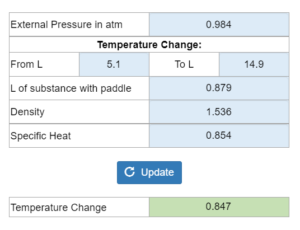
The gas in a cylinder expands from an external pressure of x atm. The volume changes from x liters to x liters. This turns a paddle in x liters of a substance. Density and specific heat are also given. You want to know the temperature change.
Experts Have Solved This Problem
Please login or register to access this content.