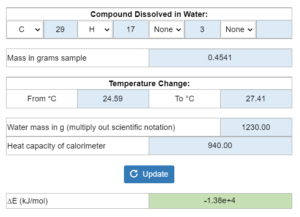
E5.019 – Heat capacity of bomb calorimeter / Activity Take: Question – Using a Constant Pressure Calorimeter to Determine the Change in Energy for a Combustion Reaction
In an experiment x grams of a sample is burned in a bomb calorimeter with x grams of water causing a temperature change. The heat capacity of the calorimeter and a balanced reaction are given. What is the heat capacity of the calorimeter?
Experts Have Solved This Problem
Please login or register to access this content.