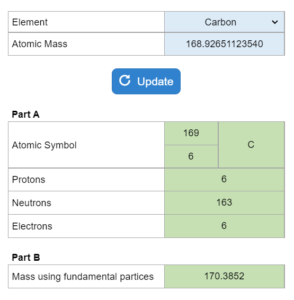
E2.030 – Atomic Symbol & Masses of the fundamental particles
The mass of an isotope is given. This is a three part questions involving: the atomic symbol, how many protons neutrons and electros, the masses of the fundamental particles and which mass is larger.
Experts Have Solved This Problem
Please login or register to access this content.