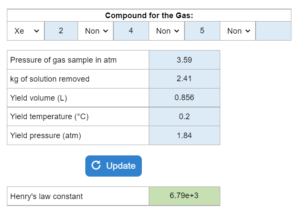
Water is mixed with a gas at a certain pressure in atm. X kg of the solution is removed and results in a volume (L), temperature (°C) and pressure (atm). What is the Henry's law constant?
Experts Have Solved This Problem
Please login or register to access this content.