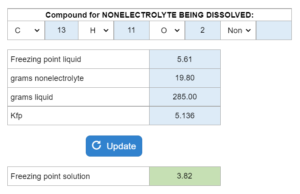
The normal freezing point of a liquid is given. X grams of a nonelectrolyte if dissolved in x grams of the liquid. Calculate the freezing point. Kfb is also given. The normal freezing point of a liquid is given. X grams of a nonelectrolyte if dissolved in x grams of the liquid. Calculate the freezing point. Kfb is also given.
Experts Have Solved This Problem
Please login or register to access this content.