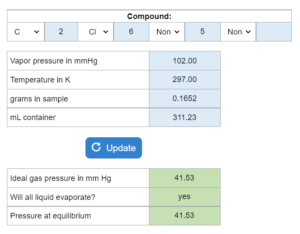
E11.004- Vapor Pressure: Evaporation or Condensation – Will all liquid evaporate? Copy
Multiple part question asking for 2 or 3 of the following: ideal pressure in mmHg, pressure at equilibrium in mmHg and/or will all liquid evaporate? Vapor pressure (mmHg), temperature (K), grams and volume (mL) are given.
Experts Have Solved This Problem
Please login or register to access this content.