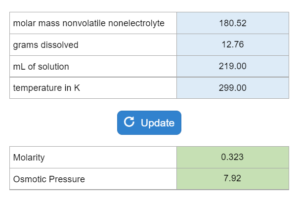
E13.013 – Osmotic Pressure Calculations (Molarity and Osmotic Pressure)
The molar mass of a nonvolatile nonelectrolyte is given. X grams of that compound are dissolved in x mL of a substance at x Kelvins. Determine the Molarity of the solution and the osmotic pressure in atms.
Experts Have Solved This Problem
Please login or register to access this content.