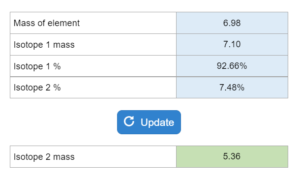
Mastery – Average Atomic Weight: Mass of Second Isotope
An elements atomic weight is given as well as % abundance of two isotopes. One isotope the mass is given, you want the mass of the second isotope.
Experts Have Solved This Problem
Please login or register to access this content.