Tutor – A student determines the heat of dissolution
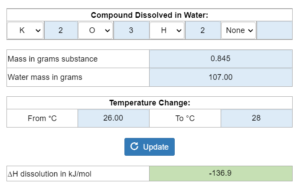
X grams of a compound are dissolved in x grams of water to cause a temperature change. There is no heat capacity of the calorimeter in this one. What is the enthalpy of dissolution (∆H dissolution)?
Experts Have Solved This Problem
Please login or register to access this content.