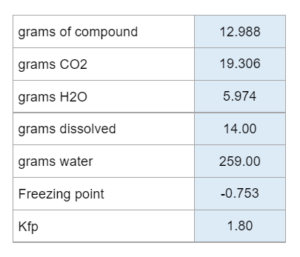
Complete combustion of x grams yield x grams CO2 and x grams H2O. Grams of the compound dissolved in grams of water as well as the freezing point and Kfp are also given. Determine the molecular formula.
Experts Have Solved This Problem
Please login or register to access this content.