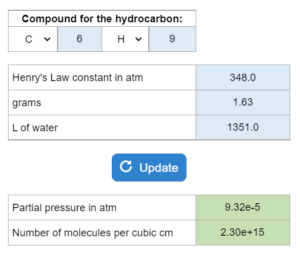
E13.032 – Henry’s law is important in environmental chemistry…
The Henry's law constant for a hydrocarbon is given in atm. A solution has x grams of the hydrocarbon per x L of water. Calculate the partial pressure and number of molecules per cubic centimeter.
Experts Have Solved This Problem
Please login or register to access this content.