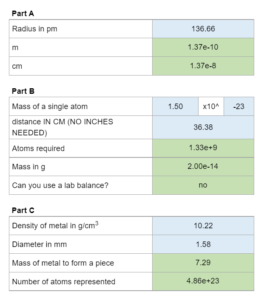
A three part question involving the following: convert pm to m and cm, determine toms required, mass in g used, lab balance usage, calculate mass using volume of a cylinder, and number of atoms represented.
Experts Have Solved This Problem
Please login or register to access this content.